Seward Lab: Current Projects
Immunotherapy
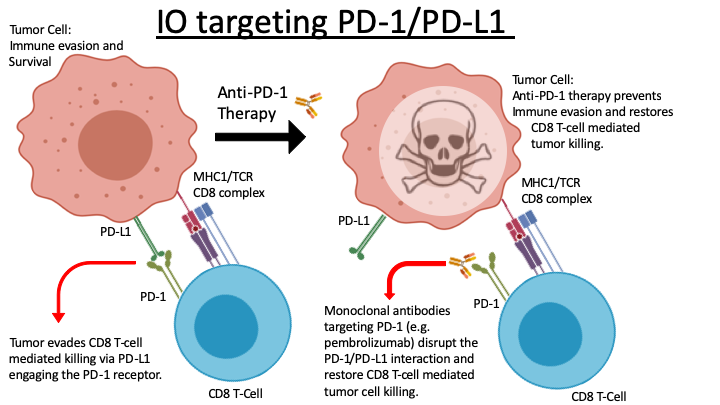
Lung cancer is the leading cause of worldwide cancer-related mortality. Despite years of dedicated work focused on improving treatment and care, the overall prognosis for patients with lung cancer remains poor. Contributing to this frustrating lack of progress is the uncomfortable fact that “lung cancer” is not a single disease. One can convincingly argue that at the genomic level every lung cancer is unique. This underlying diversity helps explain why surgical resection remains our single most effective intervention. Unfortunately, surgical resection does not guarantee cure, and further it is not always a viable clinical option depending upon disease location, disease burden or other comorbidities. For those patients with advanced disease, platinum-based chemotherapy is standard first-line treatment, but its use is often accompanied by intolerable side effects. Tumors harboring one of a small number of well-characterized and druggable oncogenic mutations have demonstrated improved initial responses with targeted therapies. However, these patients inevitably acquire therapy resistance resulting in overall 5-year survival rates that remain near 15%. Emerging therapies, including immuno-oncology strategies (IO), are seeing increased clinical use and remain under active investigation with the promise to improve treatment outcomes in lung cancer patients. IO is a broad term referring to interventions that enable/support the patient’s own immune system in recognizing and clearing tumor cells. In reality this is an activity the immune system is already adept at executing. Therefore, when a tumor becomes clinically symptomatic this generally indicates it has found a way to effectively evade the patient’s immune system. Current FDA-approved IO approaches target mechanisms known to manifest in immune-evasion. One common strategy employed by tumors to achieve this involves upregulation of programed death ligand 1 (PD-L1). PD-L1 is expressed on the surface of tumor cells where it interacts with the PD-1 receptor, expressed on cytotoxic CD8 T-cells. This interaction prevents the T-cell from destroying the tumor. Pembrolizumab (aka KEYTRUDA®) and nivolumab (aka OPDIVO®) are monoclonal antibodies that disrupt the PD-L1/PD-1 interaction. This in turn prevents the tumor cell from escaping the T-cell’s ability to attack and kill it (Figure 1). While this treatment strategy marks a major advance in oncologic care, evidenced by James P. Allison’s and Tasuku Honjo’s 2018 Nobel Prize in Physiology or Medicine, we still lack tools that reliably predict which patients will benefit from this treatment approach. By way of example, ~30% of non-small cell lung cancer (NSCLC) patients are expected to respond to current IO regimens, but we cannot reliably identify this group prior to treatment. This knowledge gap is a critical hurdle limiting efficient use of current IO and impeding development of next-generation interventions. Further, these observations underscore a demonstrable need to develop biomarkers able to differentiate which patients will respond to current IO options, while simultaneously reinforcing the need for additional approaches to address the 70% non-responder rate. Our work addresses this knowledge gap directly and aims to establish biomarkers that predict IO sensitivity while also identifying exploitable pathways that will synergize with current IO approaches, including anti-PD1 antibody therapy.
- Lung Cancer
- Immunotherapy
- Cancer metabolism
- Functional Genomics
- Epigenetics
- Aging
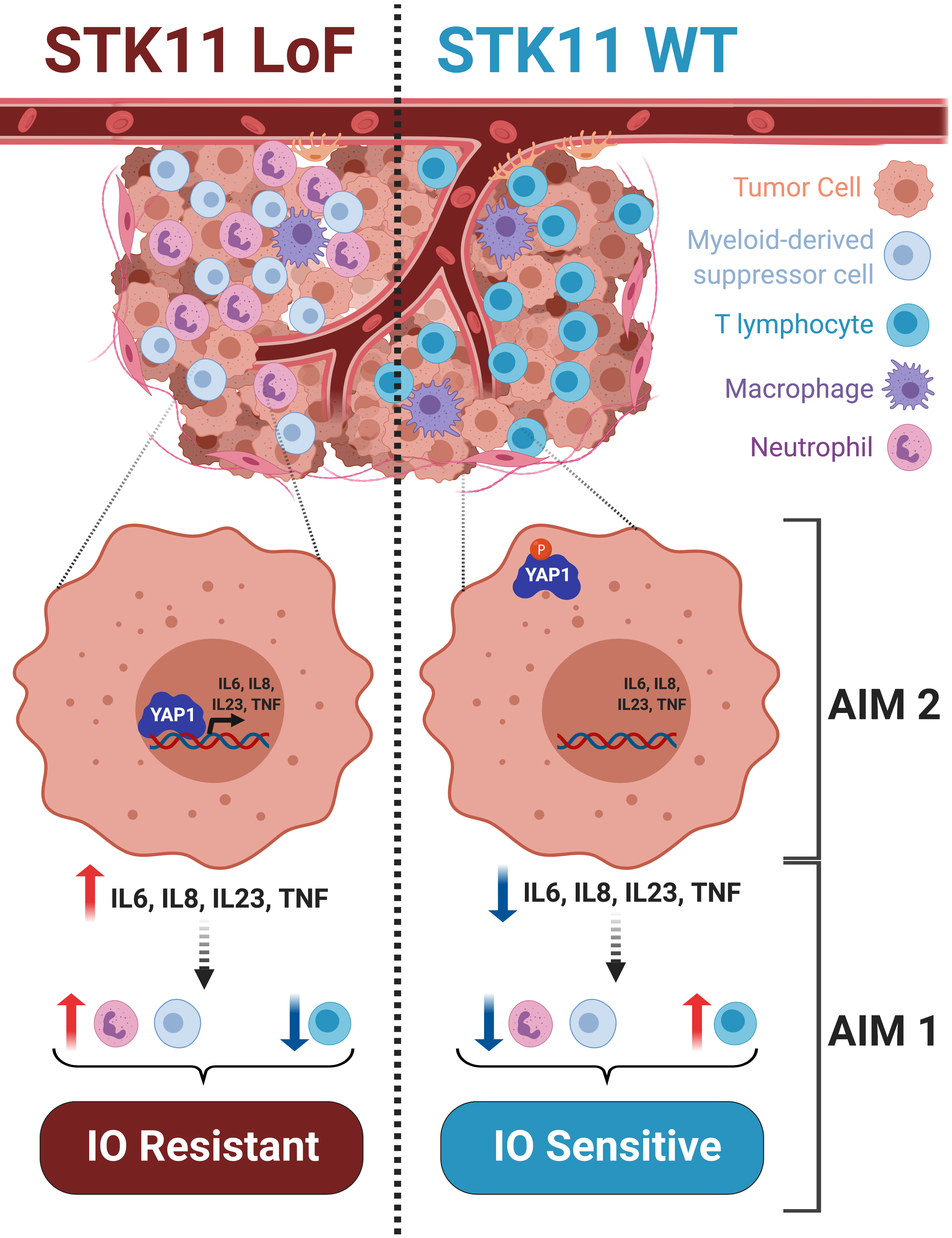
Graphical Abstract
Last modified September 19 2017 11:59 AM
